SR
Reactor working principle
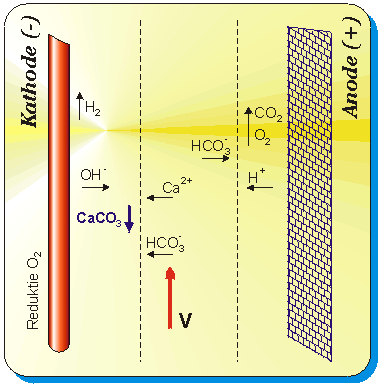 The
SR's operation based upon an electrolytic process in the reaction tank, due
to the current created between the electrodes and the reaction tank. This current,
causes to create negative ions in the proximity of the tank walls, resulting
in an increased pH. The increased pH, accelerates the calcium carbonates to
be accumulated as a deposit of the reaction tank walls.
The
SR's operation based upon an electrolytic process in the reaction tank, due
to the current created between the electrodes and the reaction tank. This current,
causes to create negative ions in the proximity of the tank walls, resulting
in an increased pH. The increased pH, accelerates the calcium carbonates to
be accumulated as a deposit of the reaction tank walls.
The increased pHaccelerates the process
of accumulation of carbonates as a deposit on the reactor wall surface.
After a certain period, the controller
will reverse the polarity to get rid of those deposits, and flushes them to
the drain.
Cathodic reactions:
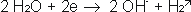
The cathode supplies electrons to
the solution. Water will be split up in H2-gas, which escapes, and OH--ions,
which react further. Bicarbonate will be formed to Carbonate:
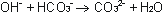
The Carbonate forms then together
with the Calcium-ions a insoluble precipitation:
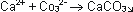
Anodic reactions:
The anode takes electrons from the
solution and divides water in H+ and Oxygen, which escapes.
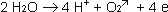
As a result of this reaction with
the formed H+, Bicarbonate will be transformed to CO2.
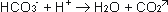
The Calcium carbonate deposits at
the cathode and is removed by changing the polarization and flushing the reactor
tank.
 The
SR's operation based upon an electrolytic process in the reaction tank, due
to the current created between the electrodes and the reaction tank. This current,
causes to create negative ions in the proximity of the tank walls, resulting
in an increased pH. The increased pH, accelerates the calcium carbonates to
be accumulated as a deposit of the reaction tank walls.
The
SR's operation based upon an electrolytic process in the reaction tank, due
to the current created between the electrodes and the reaction tank. This current,
causes to create negative ions in the proximity of the tank walls, resulting
in an increased pH. The increased pH, accelerates the calcium carbonates to
be accumulated as a deposit of the reaction tank walls.